Various floating lifeforms can use a combination of lighter than air gases, updrafts and aerodynamic lift to permanently remain in the air. Despite these differences the common feature to all of them is the balloon.
Obviously the most important part of a gasbag is the lifting gas, which leads to two questions:
- What is the lifting gas?
- How does the gasbag acquire the lifting gas?
Equally obviously, the second most important part of a gasbag is the membrane that contains the lifting gas. This was partially explored in the initial article on the physics of gasbags though there are again two important questions:
- What is the membrane made of?
- What happens if it leaks?
A third consideration is how much control the gasbag has over the the gas which also leads to two questions:
- Can the gasbag store excess lifting gas in a different form?
- Can it control the internal pressure?

Lifting gas
composition
There are various possible lifting gases and their effectiveness is linked to density. The lift they generate is the density difference in comparison to the surrounding air. On Earth air has a density of 1.2754 kg/m3 for example.
| Lifting Gas | Density (kg/m3) | Lift (kg/m3) | Lift Relative to H2 |
|---|---|---|---|
| Vacuum | 0.000 | 1.275 | 108% |
| Hydrogen (H2) | 0.090 | 1.186 | 100% |
| Helium (He) | 0.179 | 1.097 | 93% |
| Methane (CH4) | 0.716 | 0.559 | 47% |
| Ammonia (NH3) | 0.769 | 0.506 | 43% |
| Steam (H2O) | 0.804 | 0.471 | 40% |
Hydrogen is similar in effectiveness to a vacuum or helium filled gasbag. However, a vaccum gasbag is not very feasible and helium would be difficult to acquire and process. In contrast, methane or ammonia are somewhat plausible though they would produce less than half the lift of a hydrogen filled gasbag. Lift could certainly produce and process both though. Steam is an interesting option but the requirement for high temperatures renders this a little awkward for typical organic lifeforms.
source
I’ll therefore assume that hydrogen is the most likely lifting gas, so the next question is where does the gasbag acquire hydrogen from? There are three broad possibilities:
- Harvest it from the environment
- Steal it from another gasbag
- Produce it internally
Harvesting it from the environment is not viable on an Earth-like planet, but on one with an atmosphere rich in hydrogen (e.g. a gas giant) this could be possible. A filtering process would be required to separate the hydrogen from the other atmospheric constituents to increase the hydrogen concentration. Since hydrogen is a small molecule this could be achieved with a porous filter that allows hydrogen to pass but blocks larger molecules. Interestingly, since respiration in a hydrogen-dominated atmosphere might use hydrogen rather than oxygen the balloon could be linked to a gasbag’s lungs in some way.
Stealing it from another gasbag is definitely a possibility. Presumably any gasbag predators that also eat other gasbags would aim to gain hydrogen, carbon and energy from their prey. How they would adapt to “ingest” the hydrogen without losing it is an interesting question.
However, the most likely route to acquiring hydrogen for at least the base layer of the ecosystem is to produce it internally. There are several plausible ways of achieving this and conveniently the process of using microorganisms to produce biohydrogen has been studied extensively. Not only could it help mitigate global warming but it also provides inspiration for speculative evolution!
- Fermentation
- Photolysis
- Photofermentation
- Hydrogenic photosynthesis
- Hydrogen sulfide decomposition
Fermentation
Fermentation is a simple approach whereby glucose and water are used to produce organic chemicals and hydrogen. Conveniently this also produces energy, so it really only requires the gasbag to capture the hydrogen it is already producing. Various different chemical reactions are possible.
C6H12O6 + 2H2O → 2CH3COOH + 2CO2 + 4H2
C6H12O6 + 2H2O → 2CH3COOH + 2COOH + 2H2
Photolysis
Since a fundamental part of oxygenic photosynthesis is the splitting of hydrogen ions from water then it is unsurprisingly that photosynthetic algae can also produce hydrogen. This process is known as photolysis and if it is performed directly it does not produce biomass as the energy harvested from sunlight is only used to split water.
2H2O → O2 + 2H2
Photofermentation
Indirect photolysis is also possible where photosynthesis is used to produce glucose as normal but then fermentation is used to convert the glucose into hydrogen. This process is also known as photofermentation.
6CO2 + 6H2O → C6H12O6 + 6O2
C6H12O6 + 6H2O → 6CO2 + 12H2
Hydrogenic Photosynthesis
It is theoretically possible for photosynthesis to produce biomass from methane and such a process has been predicted to be common in hydrogen-dominated atmospheres. This process could be performed on other planets but a source of methane would need to be found.
CH4 + H2O → CH2O + 2H2
Hydrogen Sulfide Decomposition
It is also potentially possible to split hydrogen sulfide into sulfur and hydrogen. This has been investigated as a way of simultaneously removing sulfur from the output of fossil fuel power plants and also producing hydrogen gas as a fuel. This method would require an additional source of energy though as it does not use sunlight like the other possibilities (except fermentation).
8H2S → S8 + 8H2
Membrane
Material
The first article in the gasbag series discussed various possible membrane materials and how their density per unit area can limit the minimum size of a gasbag. These and other materials used on airships are included for comparison below for a hydrogen filled gasbag in an Earth-like atmosphere.
| Material | Area Density (g/m2) | Minimum Radius (cm) |
|---|---|---|
| Graphene | 0 | 0.0 |
| Soap Bubble | 1 | 0.3 |
| Single Cell | 10 | 2.5 |
| Mylar | 25 | 6.3 |
| Thin Skin | 50 | 12.7 |
| PVC Coated Nylon | 168 | 42.5 |
| PVC | 215 | 54.4 |
For small gasbags it is therefore vitally important that light membrane materials are used. Without this it is impossible to float without the aid of updrafts (as described in the aeroplankton article). This is of particular interest when defining the foundation of an aerial ecosystem as this is likely to involve small floating organisms. Also note that “small” gasbags in a Jupiter-like atmosphere are actually very large (i.e. bigger than a person).
However, once these minimum sizes have been exceeded by some margin the membrane material becomes less important. At that point the skin can be assumed to thicken with size and more closely resemble the skin of terrestrial animals.
Graphene is certainly worth noting as a “magic” wonder material that might be key to “realistic” gasbags. There is currently no known way for it to be produced biologically so some speculation is required. It’s amazing strength to weight ratio combined with its impermeable to gases makes it ideal for gasbags though. In addition, it potentially has other relevant capabilities:
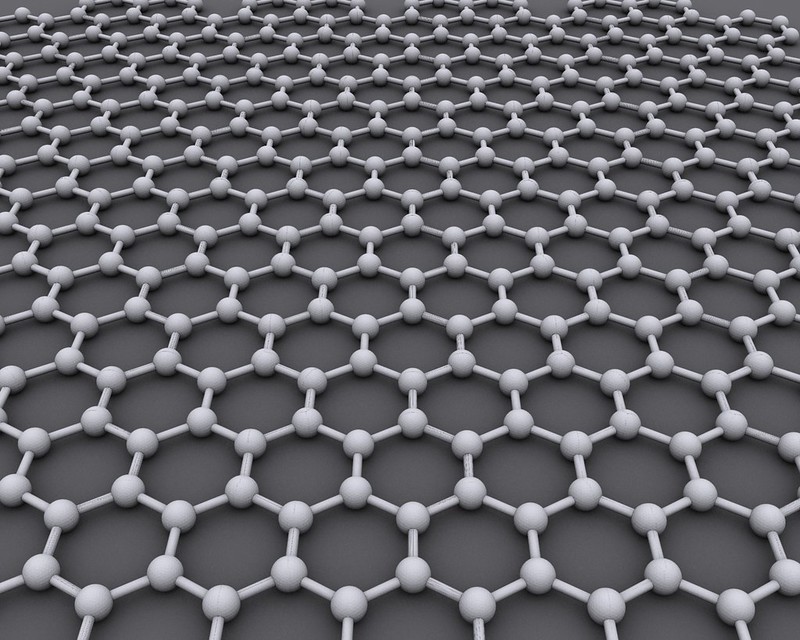
Leaks
When the skin on a terrestrial animal is punctured there is a leak of bodily fluids that has to be stopped and the skin needs to be repaired. In addition, moisture is naturally lost through the skin and needs to be replaced.
The same is true on a gasbag but the situation is more challenging as the skin must be thin to save weight and small lighter-than-air gas molecules leak out more easily, even in the absence of punctures.
It may be interesting to consider some of the following questions:
- How permeable is the membrane to the lifting gas inside?
- How permeable is the membrane to the atmosphere outside?
- Is the gasbag made of compartments so that large punctures are not fatal?
- Can the membrane self heal fast enough to prevent small punctures?
In practice it is likely that small gasbags are simply fragile and a puncture causes them to die. Even if they could repair the membrane quickly it would take a lot of energy to replace the lifting gas.
In contrast, larger gasbags would probably have a range of adaptations to survive this situation. They would perhaps have multiple internal components, combined with rapidly self healing membranes. They would potentially also store lifting gas so that they could replace lost gas quickly.
Gas Control
Storage
This leads to ask whether a gasbag can store excess hydrogen in some form so that it can be released (or absorbed) when required? This may be to replenish any lost to leaks, to increase the balloon volume or to increase the density of the balloon to control buoyancy. The swim bladder in fish performs a similar function by extracting oxygen from the blood stream to increase the bladder’s volume and adjust buoyancy.
A method used on early airships and to inflate balloons today is to react calcium hydride with water to produce calcium hydroxide and hydrogen gas.
CaH2 + 2 H2O → Ca(OH)2 + 2 H2
While this approach could work for emergencies its not possible for a gasbag to carry 100% of its hydrogen requirements in this form. In an Earth-like atmosphere, to completely replace all its hydrogen a gasbag would need to devote 78% of its body mass to calcium hydride. It would then need to react this with 67% of its body mass in water. That’s problematic unless the gasbag is immersed in water.
Storing a fraction of the total hydrogen requirement to replace lost lifting gas is viable though. The gasbag could even ditch the resulting calcium hydroxide as ballast to reduce its mass and therefore its hydrogen requirements. This would be a strategy of last resort though as replacing the calcium would be difficult in the nutrient poor aerial environment.
Note that this strategy becomes more viable if the atmosphere consists of heavier gases than on Earth as then more lift is produced from the same amount of hydrogen. Increased atmospheric pressure doesn’t help though as more hydrogen is needed inside the balloon to prevent it’s collapse.
Pressure
This leads to the final aspect mentioned at the start, the pressure of the gas within the balloon. This is important as the balance of internal and external pressure is a key factor in the structural rigidity of the balloon. There are two main possibilities:
- Variable-volume: The internal pressure matches the external pressure by changing the balloon volume.
- Superpressure: The internal pressure is always higher than the external pressure.
The first option requires a membrane that can unfold and/or stretch to accommodate the change in gas volume. Weather balloons typically operate like this and have a tall thin “flabby” shape when released before expanding to a more traditional balloon shape at high altitude as pressure decreases. The second option was described in the hybrid gasbags article with the example of the pumpkin shaped superpressure balloons used for high altitude data collection.
While either approach can work, a more sophisticated organism would want to dynamically control the aerostatic lift to allow vertical altitude control. This can be achieved through the use of ballonets. These are balloons of lifting gas within the outside envelope which contains only ambient atmospheric air. By inflating the ballonets this increases lift and vice versa. A similar result can be achieved by decreasing or increasing the pressure within a lifting gas balloon to adjust the density.
The full control of such a system is as complicated as it is for a real airship and further detail is unnecessary. However, the ability to control the amount of lift does allow an interesting form of locomotion. Clearly it can be used to control vertical motion but it can also be used to produce horizontal motion.
Underwater gliders are torpedo shaped autonomous vehicles with hydrofoils (i.e. “wings”). By inflating a bladder with oil that is lighter than water they can control their buoyancy. Just like airborne gliders, if they have negative buoyancy they will sink but the flow of water over their hydrofoils will produce a forward thrust. Unlike an airborne glider, they can then change to positive buoyancy and continue to produce forward thrust while they ascend. This produces energy efficient forward motion while they oscillate up and down in the water.
A 15 m long and 120 kg mass prototype of an airship using this variable-buoyancy propulsion has been built in the UK to demonstrate the concept. Clearly, this could also be used by gasbags to produce forward motion as they ascend and descend in the air.
Conclusions
To produce a realistic gasbag there are many considerations, though addressing them all might be a bit extreme. However, the three areas described above are perhaps worth considering when designing lighter than air life. It is certainly not a comprehensive survey but hopefully it provides a flavour of what is possible.
In particular, in most cases water is the ultimate provider of the hydrogen atoms used to produce the lifting gas. This may suggest that the collection of water is a key challenge for gasbags to overcome. Perhaps this leads to adaptations similar to desert life to continually capture water vapour, though I’ll leave that for someone else to consider.
The next article in the series will be the final article as I try to pull everything together an describe a plausible aerial ecosystem on the world of Epimetheus.
Acknowledgments
Finally, once again, thank you to Richard Bizley (bizleyart.com) for providing some of the images used in these posts about gasbags. Check out his web site for other pictures that might provide inspiration and perhaps even buy a print too.

Great series as a layman think you did a good job explaining buoyancy . One idea I find interesting is alien plants using gasbags to support themselves but still tethered to the ground like aerial equivalents of bladderwrack. Could be like a more realistic version of the beanstalk from jack and the beanstalk.
Thanks. Yes, that’s something I had considered as well. I had also wondered whether it provided a route towards a hybrid gasbag that uses both lift and aerodynamics via an intermediate stage that resembles a kytoon (i.e. a kite balloon that is tethered but still contains lifting gas).
Sounds kinda like a sea squirt like lifecycle. I wonder what the optimal shape of such floating tree/vine equivalents would be? Also I kinda love how that nasa balloon happens to look like a pumpkin to me at least.
I think that the NASA balloons should be painted orange to increase the resemblance to pumpkins. This would make them easier to find when they land too. After all “black” boxes on planes are actually orange.
The Allsopp Helikite is a useful example. It’s basically an oblate spheroid with a kite sail attached. If you’ve already justified a simple tethered gas bladder for aerostatic lift, it shouldn’t be difficult to justify a sail growing from that to produce aerodynamic lift too.
https://www.helikites.com/aerodynamic-design
Hello abbydon id like to discuss how plausible my ideas for a gasbag are for my spec evo project
Apologies for the very long delay but my life has been busy in various ways leading me to neglect this blog (and also something turned off the comment notifications). If it’s not too late then let me know I and I’ll see if I can help.